ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
AP Videos 1345 videos
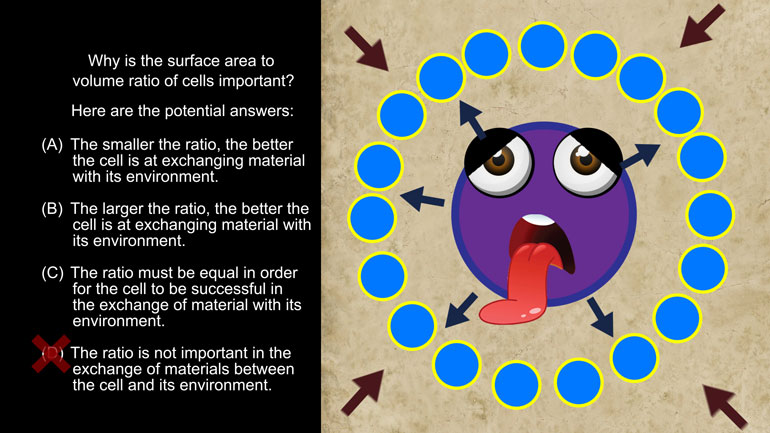
Take a look at this shmoopy question and see if you can figure out which device the speaker employs the most.
We're not going to give you a speech about how answering this Shmoopy AP English Lit question will help you succeed in life, but if we did, we wond...
AP English Language and Composition 3.5 Passage Drill. How is "forcible" being used here?
AP Chemistry 3.1 Laws of Thermodynamics 18 Views
Share It!
Description:
AP Chemistry 3.1 Laws of Thermodynamics. What is the change in enthalpy of this reaction?
Transcript
- 00:04
And here's your Shmoop du jour, brought to you by Delta H. [Plane flying]
- 00:08
Sounds like something cool and air force-y, but it’s just science.
- 00:12
Which is also super cool and exciting, right?
- 00:14
…Right?
- 00:15
Thank you. [Scientist working in a lab and explosion occurs]
Full Transcript
- 00:16
Geez, what does it take around here to get an explosion...
- 00:18
Okay, here's today’s question.
- 00:19
Using Hess's law, what is the change in enthalpy for the following reaction?
- 00:26
And here are your potential answers.
- 00:32
So the question tells us to use Hess’s law, which, unfortunately, has nothing to do with
- 00:36
avocados. [Hess kicks an avocado]
- 00:37
That’s Hass
- 00:39
Hess’s law comes from the fact that the overall enthalpy change of a reaction doesn’t
- 00:44
depend on the reaction pathway, or how we get from reactants to products.
- 00:50
For example, we could react one mole of A and 2 moles of B to produce 2 moles of C.
- 00:54
Let’s call this Peter. [Formula example of Hess's law]
- 00:56
What, you think all reactions want to be called "reaction"?
- 00:59
Do you just want to be called "human six billion nine hundred and sixty-two thousand"?
- 01:04
No.
- 01:05
We're calling "reaction 1" Peter.
- 01:07
We could also react one mole of A with one mole of B to produce two moles of D, then
- 01:14
react these moles of D with one mole of B to produce two moles of C.
- 01:19
We’ll call these reactions Sarah and Phil, because they're more than just 2 and 3.
- 01:25
As you can see, reactions Sarah and Phil can be added to produce reaction Peter.
- 01:31
The overall reaction is the same in both cases—one mole of A and two moles of B produce two moles
- 01:37
of C.
- 01:39
So in the same way that we added the actual reactions, we can add the enthalpy changes.
- 01:44
Hess’s law says that the overall enthalpy change shouldn’t depend on the reaction
- 01:49
path chosen.
- 01:51
That means that the enthalpy change for Peter should equal the sum of the enthalpy changes [Hess's law for Peter, Sarah, Phil]
- 01:56
for Sarah and Phil.
- 01:58
Okay, so back to the problem at hand! [Man in a rocking chair]
- 02:00
And we’re not referring to the fact we're naming reactions and may be having a mental
- 02:04
breakdown. [Man crying in a rocking chair]
- 02:05
We need to find a way to add the three reactions for which we have ?H to produce the top reaction.
- 02:11
Here’s what we came up with.
- 02:13
If we multiply the first equation by one half, reverse and multiply the second equation by
- 02:18
3/2, and multiply the third equation by one half, then add them all up, we get the first [All equations added together]
- 02:25
reaction.
- 02:26
We add the ?H values in the same way.
- 02:29
Just don’t forget that if you reverse a reaction, you have to negate the ?H value,
- 02:34
and if you multiply a reaction by a factor, such as 1/2, you should multiply the ?H value [Man discussing ?H value]
- 02:39
by that number too.
- 02:41
So chugga chugga chug... chug through the math machine and…
- 02:46
Looks like our answer is B, 886 kJ.
- 02:50
Now it's time to brainstorm the pilot episode of our new sitcom. [People sitting on a couch watching a sitcom]
- 02:53
It's about three young reactions living together…we're calling it, "Three's Company, but with Reactions".
- 02:58
…We're not great at naming things.
Related Videos
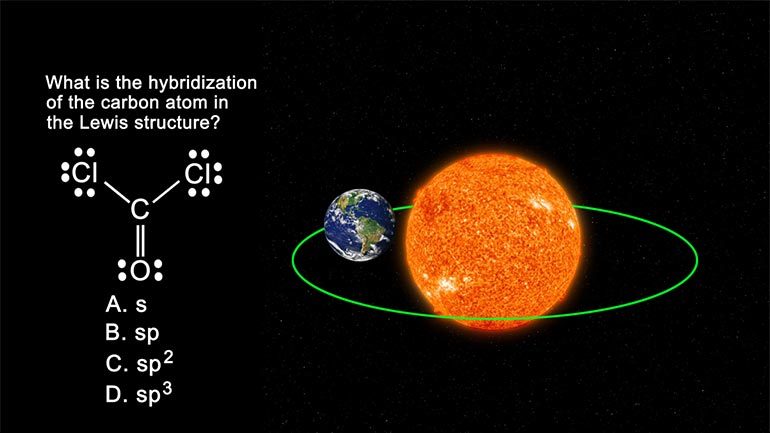
AP Chemistry: Structure of Atoms Drill 1, Problem 5. What is the hybridization of the carbon atom in the Lewis structure?
AP Chemistry DBQ/Free Response. Perform the following calculations.
AP Chemistry 1.3 Chemical Reaction Rates. What is the overall order of the reaction?
AP Chemistry 1.4 Chemical Reaction Rates. What are the correct units for a second order rate constant?
AP Chemistry 1.5 Chemical Reaction Rates. What is the rate law for the reaction?