ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
AP Videos 1345 videos
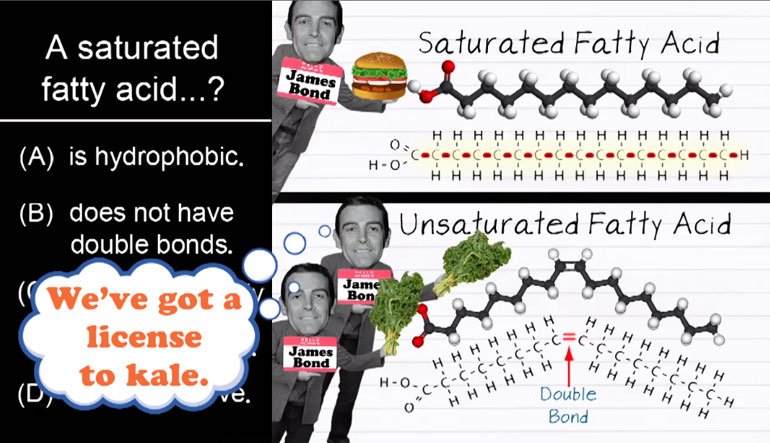
AP Biology: Biological System Interactions Drill 1, Problem 1. Complete the sentence about a saturated fatty acid.
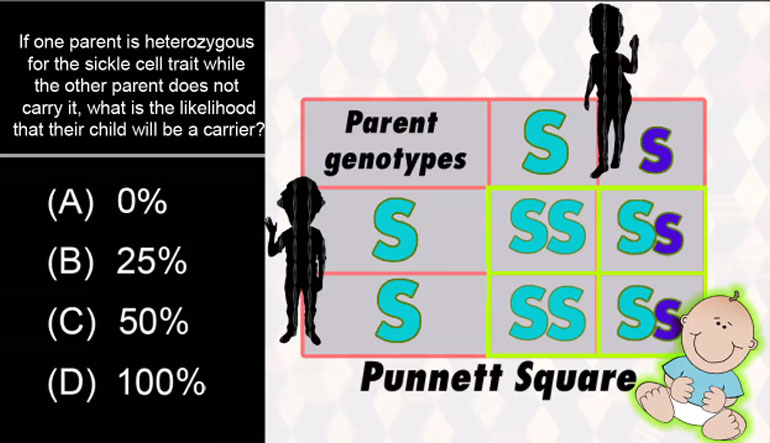
AP Biology: Essential Life Process Information Drill 1, Problem 1. If one parent is heterozygous for the sickle cell trait while the other par...
AP Biology: Evolution Drives the Diversity and Unity of Life Drill 1, Problem 1. The first cells on planet Earth were likely what?
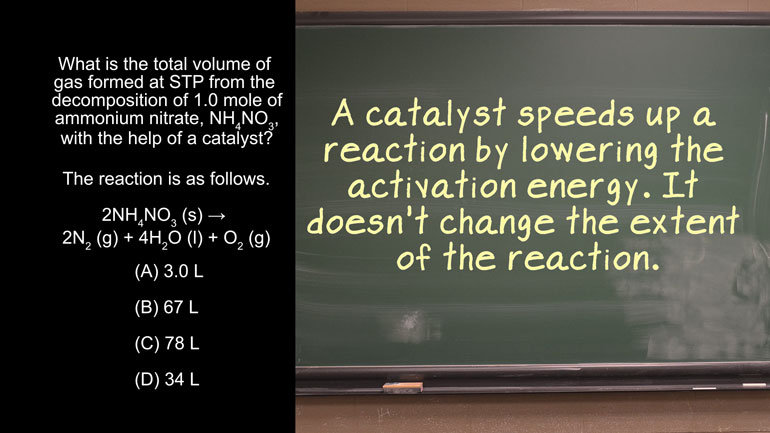
AP Chemistry 1.2 Laws of Thermodynamics 20 Views
Share It!
Description:
AP Chemistry 1.2 Laws of Thermodynamics. What is the standard enthalpy for the following reaction?
Transcript
- 00:04
Here’s your Shmoop du jour, brought to you by standards.
- 00:07
They're the difference between living in this room… [Boy playing in his bedroom]
- 00:11
And this room… Alrighty, here’s our question:
- 00:14
What is the standard enthalpy for the following reaction, given the standard enthalpies of
- 00:20
formation?
Full Transcript
- 00:21
Here's the given reaction…lots of no's in there… [Chemical reaction equation]
- 00:24
And we're given the heats of formation for NO, N2O and NO2.
- 00:29
And here are our potential answers: This reaction is saying, “No, no, no!”
- 00:38
But we’re saying, “yes, yes, yes” to finding the standard enthalpy of the reaction.
- 00:42
Heh. Classic chemistry joke… Because nitric oxide is NO…? [Man thrown banana skins at him on stage]
- 00:47
Well we thought it was classic.
- 00:49
Anyway, to solve this problem, we need to find the standard enthalpy of reaction using
- 00:53
the following relationship: The enthalpy of reaction is equal to the sum
- 00:57
of the enthalpies of formation the products minus the sum of the enthalpies of formation [enthalpy of reaction definition]
- 01:04
of the reactants. Alright, well unlike staying awake after a
- 01:08
big lunch, this is actually pretty easy. In this reaction, our products are N2O and
- 01:13
NO2. We need to look at the heats of formation for these molecules given in the problem statement [Heat formation of N2O and NO2]
- 01:18
and add them up. Next, we see that the reactants are three
- 01:21
N O molecules, so we need to subtract three times the heat of formation of N O.
- 01:26
When we do the math, we find that the enthalpy of reaction is negative 154 kJ/mol, which
- 01:33
is answer (A). And yup, that's all she wrote. We're done.
- 01:37
Now that we’ve figured out this problem, it's time to reward ourselves with a big lunch…and [Boy eating food and falls asleep]
- 01:41
maybe a nap…
Related Videos
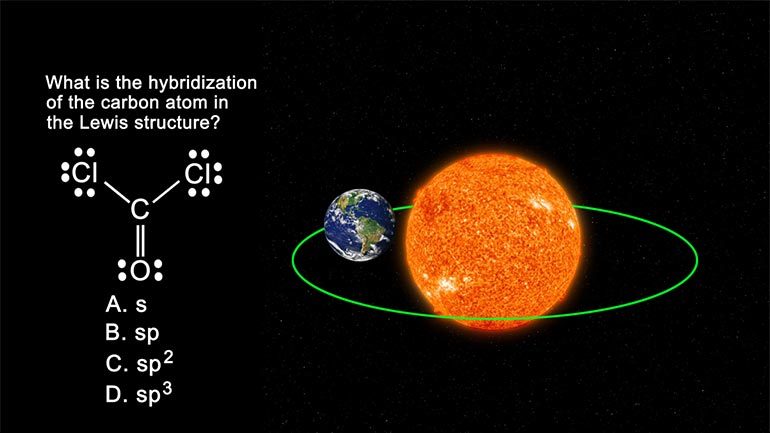
AP Chemistry: Structure of Atoms Drill 1, Problem 5. What is the hybridization of the carbon atom in the Lewis structure?
AP Chemistry DBQ/Free Response. Perform the following calculations.
AP Chemistry 1.3 Chemical Reaction Rates. What is the overall order of the reaction?
AP Chemistry 1.4 Chemical Reaction Rates. What are the correct units for a second order rate constant?
AP Chemistry 1.5 Chemical Reaction Rates. What is the rate law for the reaction?