ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
AP Videos 1345 videos
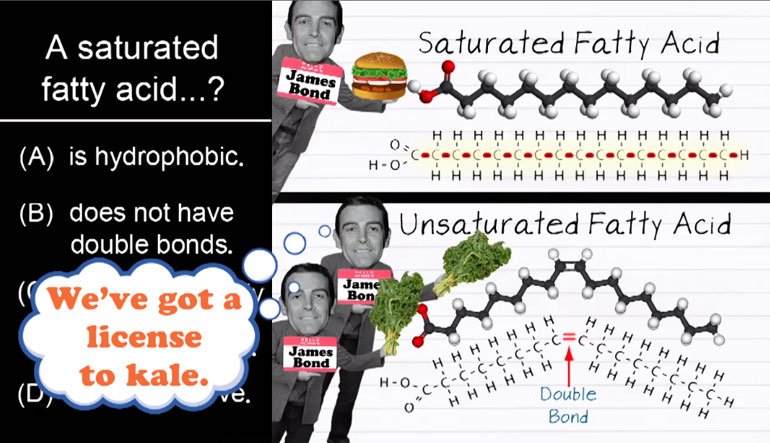
AP Biology: Biological System Interactions Drill 1, Problem 1. Complete the sentence about a saturated fatty acid.
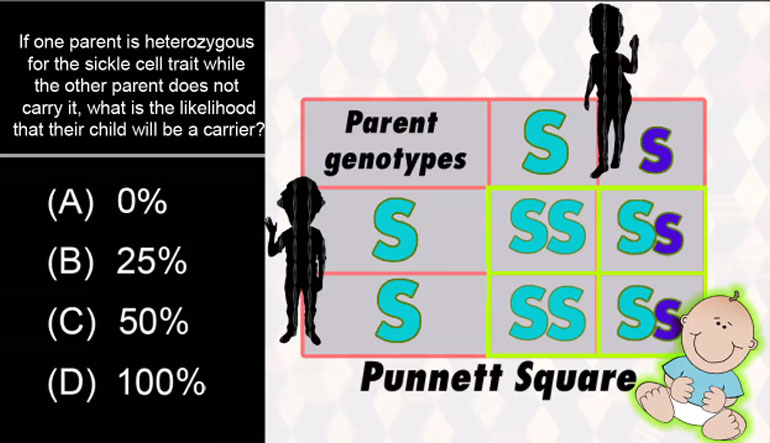
AP Biology: Essential Life Process Information Drill 1, Problem 1. If one parent is heterozygous for the sickle cell trait while the other par...
AP Biology: Evolution Drives the Diversity and Unity of Life Drill 1, Problem 1. The first cells on planet Earth were likely what?
AP Chemistry 2.1 Laws of Thermodynamics 14 Views
Share It!
Transcript
- 00:04
And here's your Shmoop du jour, brought to you by the unbreakable second law of thermodynamics. [A boy practicing karate on a book of thermodynamics]
- 00:09
Other unbreakable laws include: “Eat your vegetables” and “Sharing is caring.”
- 00:14
Okay, here's today’s question.
- 00:16
Which of the following would cause an increase in entropy?
- 00:19
And here are our potential answers. [mumbling]
Full Transcript
- 00:21
By the way, that N.O. in answer D?
- 00:25
Yeah, it’s not the opposite of “YES.” [A girl looking confused]
- 00:27
It’s nitric oxide, people.
- 00:28
That’s not to be confused with nitrous oxide, better known as laughing gas. [Students laughing in class and teacher asks what is funny]
- 00:34
But who needs laughing gas when you’ve got us, and our dazzling sense of humor?
- 00:39
Anyway, let’s get this thing going.
- 00:41
To answer this question, we probably need to know what the heck entropy is.
- 00:43
Well, according to the unbreakable second law of thermodynamics, the overall entropy [Image of a galaxy in a thermodynamics book]
- 00:48
in the universe never decreases.
- 00:51
Increasing entropy means a dispersal of energy or matter.
- 00:54
This leads to increased disorder or messiness. [A boys bedroom door opens and lots of mess inside]
- 00:58
So yes, your bedroom is messy and the Universe is, too.
- 01:03
Try that one on Mom and Dad. Good luck!
- 01:05
All right, we have to think about which of our answer choices describes an increase in
- 01:08
disorder or dispersal.
- 01:11
We know that the molecules in a gas are more spread out than in a liquid, and the molecules [Molecules in liquid bouncing up and down]
- 01:14
in a liquid are more spread out than in a solid.
- 01:17
The molecules in a gas are the most dispersed and most disordered…gases have the highest
- 01:22
entropy.
- 01:23
Okay, so let’s look at choice A. Freezing water represents a liquid to solid
- 01:27
phase change, which corresponds to decreased disorder and thus decreased entropy. [Molecules inside an ice cube]
- 01:32
So we can lose A. Those solid molecules sure are orderly, but
- 01:36
jeez, they don’t have much fun.
- 01:38
Could it be C - crystallization of salt from a supersaturated solution?
- 01:42
Well, like freezing water, this answer describes a transition from the liquid phase to the [Molecules bouncing in a wine glass]
- 01:46
solid phase, which corresponds to a decrease in disorder and thus a decrease in entropy.
- 01:52
So say bye-bye to C. Choice D describes a reaction in the gaseous [Gaseous molecules in disorderly fashion]
- 01:56
phase.
- 01:57
We have two molecules combining to form one—matter that was once dispersed is now more compact.
- 02:03
This is a decrease in disorder, and thus a decrease in entropy, and thus a decrease in
- 02:07
our interest in choice D. That leaves us with choice B, boiling water. [A pan of boiling water]
- 02:12
Boiling water represents a liquid to vapor phase change, which corresponds to increased
- 02:16
disorder and thus increased entropy.
- 02:18
That means that B is our answer.
- 02:20
Hey, now that you know your second law of thermodynamics…maybe you could – we don’t
- 02:24
know - clean your room? [boy using a vacuum in his room and mother watches]
- 02:25
Just a thought.
Related Videos
AP Chemistry 1.3 Chemical Reaction Rates. What is the overall order of the reaction?
AP Chemistry 1.4 Chemical Reaction Rates. What are the correct units for a second order rate constant?
AP Chemistry 1.5 Chemical Reaction Rates. What is the rate law for the reaction?
AP Chemistry 3.2 Laws of Thermodynamics. What is the value for ΔG?