ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
AP Chemistry Videos 54 videos
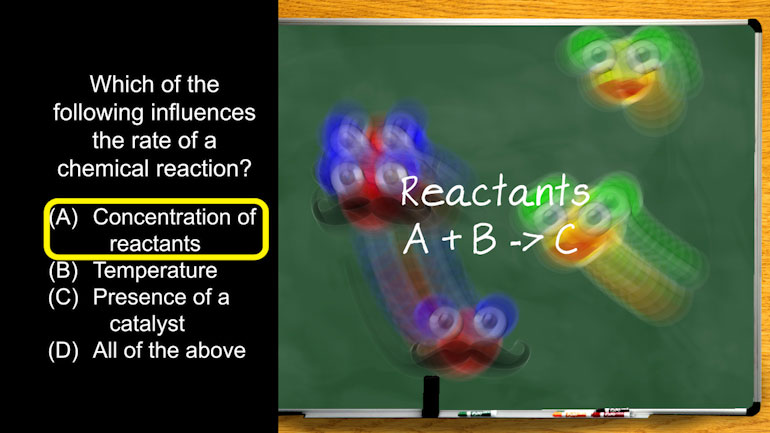
AP Chemistry 1.3 Chemical Reaction Rates. What is the overall order of the reaction?
AP Chemistry 1.4 Chemical Reaction Rates. What are the correct units for a second order rate constant?
AP Chemistry 1.5 Chemical Reaction Rates. What is the rate law for the reaction?
AP Chemistry 2.1 Rearrangements and Reorganization of Atoms 7 Views
Share It!
Description:
AP Chemistry 2.1 Rearrangements and Reorganization of Atoms. What is the percent yield for this reaction?
Transcript
- 00:04
Here’s your Shmoop du jour, brought to you by decaffeinated coffee, also known as a cruel, [Man drinks decaf coffee]
- 00:09
cruel joke.
- 00:10
Here’s today’s question:
- 00:12
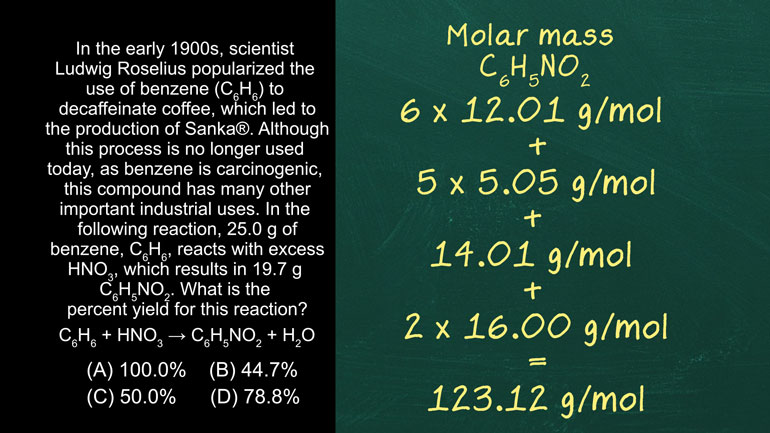
In the early 1900s, scientist Ludwig Roselius popularized the use of benzene (C6H6) to decaffeinate
- 00:21
coffee, which led to the production of Sanka®.
Full Transcript
- 00:24
Although this process is no longer used today, as, benzene is carcinogenic, this compound
- 00:30
has many other important industrial uses.
- 00:32
In the following reaction, 25.0 g of benzene, C6H6, reacts with excess HNO3, which results [Chemical reaction appears]
- 00:41
in 19.7 g C6H5NO2.
- 00:45
What is the percent yield for this reaction?
- 00:49
And here are your potential answers:
- 00:52
The first thing we should do is figure out what we’re actually being asked. [Student raises hand to answer question]
- 00:56
We have a feeling there’s a whole lot of fluff in this one.
- 00:59
We don’t care about this Ludwig van Benzthoveen guy and whatever company his blasphemously [Beethoven sipping coffee]
- 01:04
undercaffeinated coffee created, we just want to know about the reaction.
- 01:09
So the important part of the question is just the last two sentences, the equation, and
- 01:13
the answers.
- 01:14
Boom, boom, boom, cut all that unnecessary stuff out of your life. [unnecessary part of question disappears]
- 01:17
Okay, so it’s asking us about the percent yield and giving us grams going into and out
- 01:22
of a reaction.
- 01:23
And by grams, we mean the unit, not the delicious cracker. [Man takes cracker and person uses wand to make it disappear]
- 01:27
Yeah, we were disappointed, too.
- 01:28
Because this is a question involving specific amounts of chemicals reacting, before we even
- 01:32
think about how to solve it, we’re going to convert everything to moles.
- 01:36
All of our given values are in grams, so let’s convert from grams to moles by using the molar
- 01:40
mass.
- 01:41
Which means we have to calculate the molar mass.
- 01:42
The molar mass of benzene is 6 times the atomic weight of Carbon, 12.01 grams per mole from
- 01:46
the periodic table, plus 6 times the atomic weight of Hydrogen, 1.01 grams per mole, which
- 01:52
gives us 78.12 grams per mole.
- 01:56
The molar mass of C6H5NO2 is 6 times the atomic weight of carbon plus 5 times the atomic weight [molar mass appears]
- 02:01
of hydrogen plus the atomic weight of Nitrogen, 14.01 grams per mole, plus 2 times the atomic
- 02:08
weight of Oxygen, 16.00 grams per mole, which gives us 123.12 grams per mole.
- 02:14
Whew.
- 02:15
Still with us?
- 02:16
Now, let’s calculate the moles of each substance so we can stop talking about weight so much. [Benzene and nitrobenzene on balance scales]
- 02:20
It’s making the molecules self-conscious…
- 02:24
To find the moles of each substance, we take the mass in grams and divide by the molecular
- 02:29
weight.
- 02:30
This is 25.0 grams over 78.12 grams per mole for benzene, giving us 0.320 moles of benzene. [moles calculation appears]
- 02:38
For C6H5NO2, it’s 19.7grams over 123.12 grams per mole giving us 0.160 moles of C6H5NO2.
- 02:48
Time to mop your brow. [Worker wipes away sweat from brow]
- 02:50
We did it.
- 02:51
Wait… what was the question?
- 02:53
Oh right.
- 02:54
Percent yield.
- 02:55
And that’s not a matter of how often one chemical let’s another one pass at an intersection. [Car stops at a yield sign]
- 02:59
It’s how much product you got out of a reaction.
- 03:03
Specifically, how much product you actually get as a percentage of what you could have
- 03:06
gotten if everything went perfectly.
- 03:08
Because, like life, things in chemistry don’t always go perfectly. [Scientist eating a cookie]
- 03:14
In this reaction, we see the same number of benzenes and C6H5NO2 molecules on either side
- 03:18
of the reaction, so each time we use a benzene we make a C6H5NO2.
- 03:24
This means we could ideally make as many moles C6H5NO2 as we had moles of benzene to start
- 03:30
with, which we calculated to be 0.320.
- 03:32
To calculate the percent yield, we take the moles we actually got over the moles we could [percent yield equation appears]
- 03:37
have gotten, 0.160 over 0.320, and multiply by 100.
- 03:41
This gives us a 50 percent yield for the reaction.
- 03:44
Which isn’t a great result, but it is our answer.
- 03:48
If your lap supervisor is disappointed, you can offer him a cookie. [Man offers supervisor a cookie]
- 03:50
Or 20.
- 03:51
But no more.
Related Videos
AP Chemistry 1.3 Chemical Reaction Rates. What is the overall order of the reaction?
AP Chemistry 1.4 Chemical Reaction Rates. What are the correct units for a second order rate constant?
AP Chemistry 1.5 Chemical Reaction Rates. What is the rate law for the reaction?
AP Chemistry 3.2 Laws of Thermodynamics. What is the value for ΔG?