ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
AP Chemistry 3.3 Laws of Thermodynamics 4 Views
Share It!
Description:
AP Chemistry 3.3 Laws of Thermodynamics. How much energy would be required to completely melt the ice?
Transcript
- 00:04
And here's your Shmoop du jour, brought to you by iced tea…A refreshing drink turned
- 00:09
talented rapper turned even more talented detective on Law and Order SVU. [Iced tea glass transforms from rapper to detective]
- 00:13
Dun dun.
- 00:17
Alright, here's today’s question.
- 00:18
On a hot summer day, there is nothing like a nice, cold glass of iced tea.
Full Transcript
- 00:22
To cool iced tea, the ice cubes must melt.
- 00:25
How much energy would be required to completely melt 60.0 g of ice at 0 °C?
- 00:31
The heat of fusion for ice equals 6.01 kJ/mol.
- 00:36
And here are your potential answers.
- 00:39
All right, for the quantitative part of this problem, we need to think about water. [Person using a calculator]
- 00:43
Ah, so peaceful…
- 00:44
…Darn it.
- 00:45
Now we need a bathroom…[Toilet flushes]
- 00:47
Okay, where were we?
- 00:48
Oh, yeah, science.
- 00:50
Great.
- 00:51
So, we're given a heat of fusion, which is the amount of energy needed to melt one mole
- 00:54
of ice at 0°C.
- 00:55
To calculate the amount of energy needed to melt 60 grams of ice, we have to convert this [Robot shoots a laser and melts ice cubes]
- 01:01
weight to moles using the molecular weight of water.
- 01:04
Now we can just multiply the number of moles of ice by the heat of fusion to calculate
- 01:08
the overall heat, Q.
- 01:10
This question is a great example of why units are your best friend… [Boy juggling with measurement units]
- 01:13
Sorry, dogs.
- 01:14
You've been demoted.
- 01:16
By looking at the units of our answer and the information that we’ve got, we can deduce
- 01:19
the equation that should be used.
- 01:21
This is a great trick for when you’re unsure or just want to double check. [Girl scribbling in class]
- 01:25
It's also just a great party trick in general…y'know, if your thing is getting uninvited to parties. [Boy at a party with 3 upside down red cups]
- 01:30
Anyway, we can see that the correct answer is choice C, - 20 kJ.
- 01:34
All right, Fido, you can come back now.
- 01:37
We’re ready to play. [Boy shouts for Fido to come play]
- 01:39
Fido?
- 01:40
Where are you?
- 01:41
We were just kidding about that best friend thing… [Fido on the sofa reading a newspaper]
Up Next
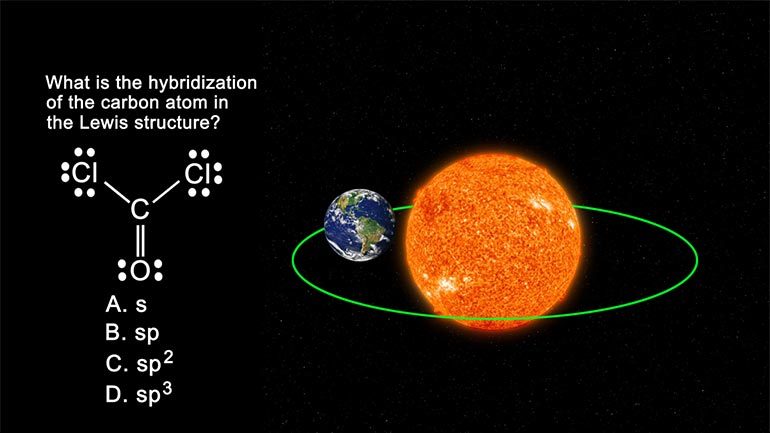
AP Chemistry: Structure of Atoms Drill 1, Problem 5. What is the hybridization of the carbon atom in the Lewis structure?
Related Videos
AP Chemistry DBQ/Free Response. Perform the following calculations.
AP Chemistry 1.3 Chemical Reaction Rates. What is the overall order of the reaction?
AP Chemistry 1.4 Chemical Reaction Rates. What are the correct units for a second order rate constant?
AP Chemistry 1.5 Chemical Reaction Rates. What is the rate law for the reaction?